Of orbitals 2. Complete this structure for anthracene C14H10 by adding bonds and hydrogen atoms as necessary.
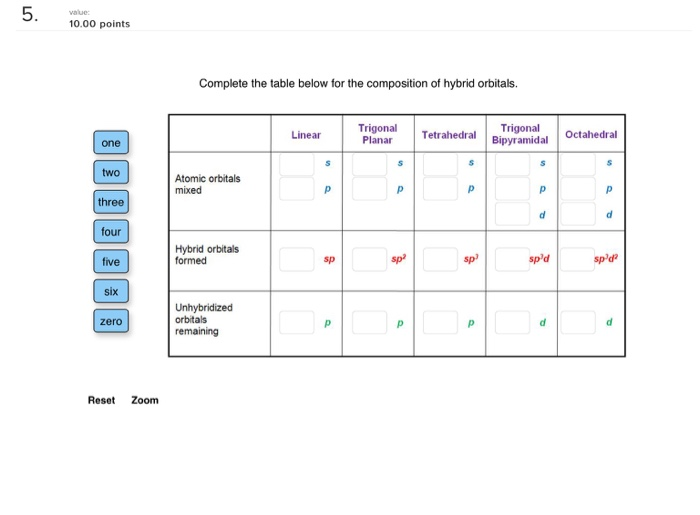
Solved 5 Value 10 00 Points Complete The Table Below For Chegg Com
They consist of four equivalent hybrid orbitals that point approximately toward the corners of a tetrahedron Figure 2.

. The carbon is bonded to two other atoms that means it needs two hybrid orbitals aka sp. This means there can be at most four sp-type orbitals as in the case of the sp 3 hybrid orbital. TrigonalT Planar TetrahedralBipyramidal Linear one Bipyramidal Octahedral two Atomic orbitals mixed three four Hybrid orbitals formed sp d five Sp sp siX Unhybridized orbitals remaining zero.
Complete the table below for the composition of hybrid orbitals. TrigonalT Planar TetrahedralBipyramidal Linear one Bipyramidal Octahedral two Atomic orbitals mixed three four Hybrid. The masses amu and abundances of the isotopes are given in the table below.
Linear Trigonal Planar Tetrahedral Trigonal Bipyramidal Octahedral one two Atomic orbitals mixed three BOBODOO four five Hybrid orbitals formed zero Unhybridized orbitals remaining Match each of the given theories with its definition Molecular Orbital MO Theory A model that attempts to. An idealized single crystal of diamond is a gigantic molecule because all the atoms are inter-bonded. Anthracene is a yellow crystalline solid found in coal tar.
Of electron pairs around the central. Use partial orbital diagrams to describe how mixing of the atomic orbitals of the central atoms leads to hybrid orbitals in each of the following. That makes three hybrid orbitals for lone pairs and the oxygen is bonded to one hydrogen which requires another sp 3 orbital.
The valence orbitals in an isolated oxygen atom are a 2 s orbital and three 2 p orbitals. Cho 1 Complete the table below for the composition of hybrid orbitals. C The molecule contains four lone pairs of valence electrons.
When both the 3 d z 2 and 3 d x 2 - y 2 orbitals are mixed with the 3 s 3 p x 3 p y and 3 p z orbitals the result is a set of six sp 3 d 2 hybrid orbitals that point toward the corners of an octahedron. This arrangement results from sp 2 hybridization the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry. The new orbitals that result are called hybrid orbitals.
The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. The first row has been completed for you. D One carbon is described by sp 3 hybridization.
The electron- group arrangement. 0 Hybrid orbitals overlap to form pi bonds Content attribution Question 6 1 point A molecule with the formula AX4E2 uses 1 sp hybrid orbitals to form its bonds 2 sp3d hybrid orbitals 3 4 Sp2 hybrid orbitals sp3d2 hybrid. Six zero Unhybridized orbitals remaining P P p d d.
View the full answer. Cho 1 Complete the table below for the composition of hybrid orbitals. OH b Sulfur tetrafluoride SF.
Hybrid orbital Sp2. Hybrid orbital Sp. Complete the table below for the composition of hybrid orbitals.
Posted one year ago. Complete the table below for the composition of hybrid orbitals. Science Chemistry QA Library Complete the table below by writing the symbols for the cation and anion that make up each ionic compound.
Complete the table below for the composition of hybrid orbitals. We then postulate the type of hybrid orbitals required and write a partial orbital diagram. If no reaction occurs write NR after the reaction arrow.
Complete the balanced molecular chemical equation for the reaction below. Hybrid orbital Sp3. However when some of the native p orbitals are empty the hybridization changes.
A One carbon is described by sp 2 hybridization. Of electron pairs around the central. The bonding has given diamond some very unusual properties.
The valence orbitals in an oxygen atom in a water molecule differ. Pauling showed that when the 3d z 2 orbital is mixed with the 3s 3p x 3p y and 3p z orbitals on an atom the resulting sp 3 d hybrid orbitals point toward the corners of a trigonal bipyramid. Complete the table below for the composition of hybrid orbitals.
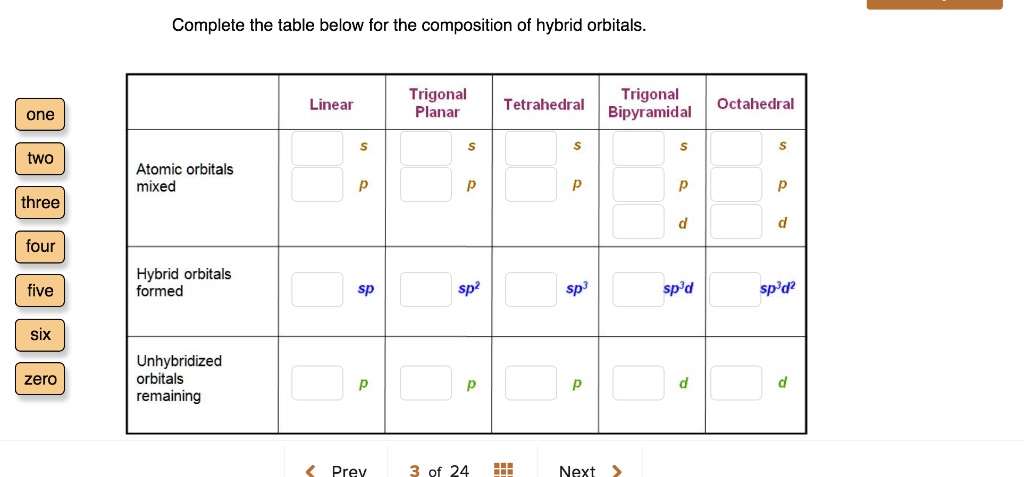
333 points Linear Trigonal Planar one Tetrahedral Trigonal Bipyramidal Octahedral eBook S S S s S References two Atomic orbitals mixed P P p p P three d d four Hybrid orbitals formed five sp sp. Up to 256 cash back O Hybrid orbitals have the same orientation as atomic orbitals. Every lone pair needs it own hybrid orbital.
Of electron pairs around the central. The average atomic mass of the element is _____ amu. Six zero Unhybridized orbitals remaining P P p d d.
221X 55700 22090g 220X 38800 22000g 218X 5500 21810g. Complete the table below for the composition of hybrid orbitals. The element X has three naturally occurring isotopes.
Draw a complete line-bond or electron-dot formula for acetic acid and then decide which statement is incorrect. Of orbitals 3. B The molecule contains only one bond.
Cr₂SO₄₃aq 3 NH₄₂CO₃aq Cr₂CO₃₃s 3 NH₄₂SO₄aq. Ionic compound cation anion Na Cl Na MaNO MnS Mnl 4 vso. O Hybrid orbitals form from combining atomic orbitals.
Complete the table below for the composition of hybrid orbitals. 333 points Linear Trigonal Planar one Tetrahedral Trigonal Bipyramidal Octahedral eBook S S S s S References two Atomic orbitals mixed P P p p P three d d four Hybrid orbitals formed five sp sp. Complete the table below for the composition of hybrid orbitals.
Trigonal Planar TetrahedralBipyramidal Linear Bipyramida ctahedral one two Atomic orbitals mixed three four Hybrid orbitals formed sp3d spd five sp sp2 six Unhybridized orbitals remaining zero. Previous question Next question. That makes 4 orbitals aka sp 3.
Trigona Tetrahedral Bipyramidal Planar Trigonal Bipvramidal Linear one two Atomic orbitals mixed three four Hybrid orbitals formed five sp Sp sp d SIX Unhybridized orbitals remaining zero Pre 3 of 24Next Question. The bond length of 154 pm is the same as the C-C bond length in ethane propane and other alkanes. Of orbitals 4.
Trigonal Tetrahedral Bipyramidal Trigonal Octahedral Linear Planar one two Atomic orbitals four Hybrid orbitals five sp six zero Reset Zoom Prey 3 of 24 Next. For these elements there is a maximum of one filled s and three filled p orbitals in the valence shell. Of electron pairs around the central.
Elements in the first two periods of the periodic table have only s or p orbitals. The bonding no doubt is due to the sp3 hybrid orbitals.
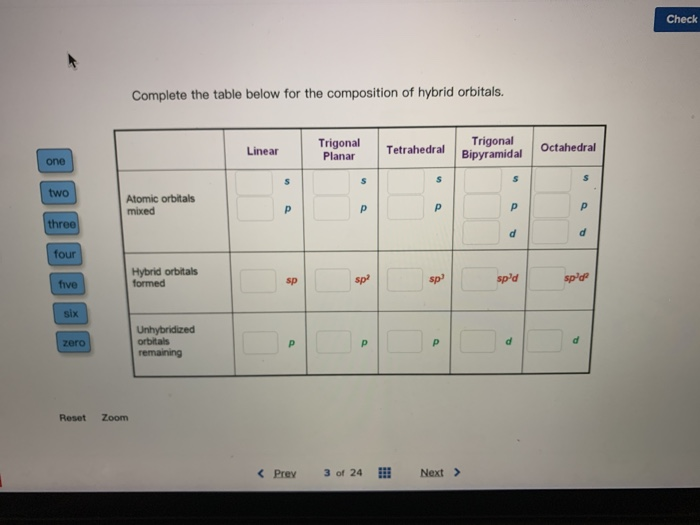
Solved Complete The Table Below For The Composition Of Hybrid Orbitals Trigonal Planar Trigonal Tetrahedral Bipyramidal Linear Octahedral One Two Atomic Orbitals Mixed Three Four Hybrid Orbitals Formed Five Sp Spd Six Unhybridized
Solved Complete The Table Below For The Composition Of Chegg Com
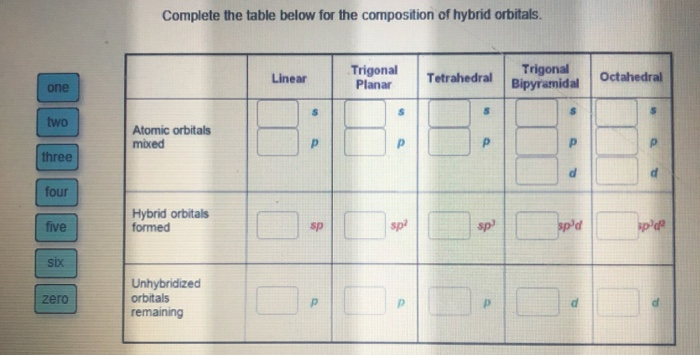
Solved Complete The Table Below For The Composition Of Chegg Com
0 Comments